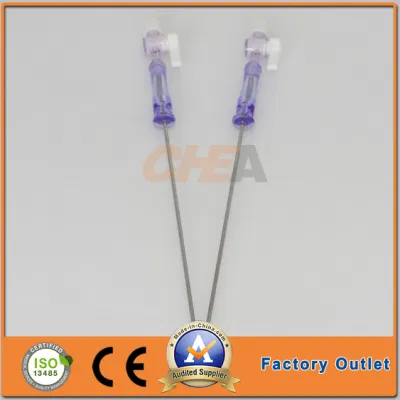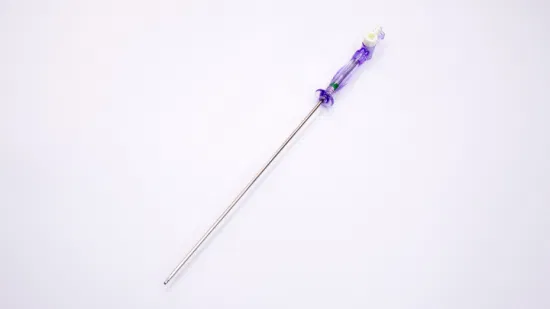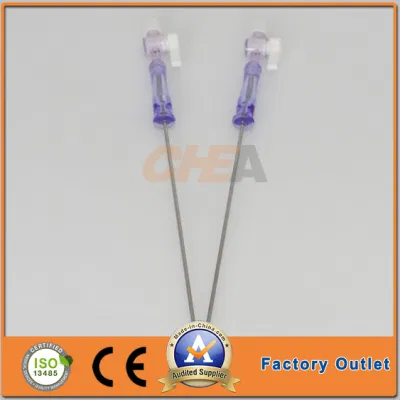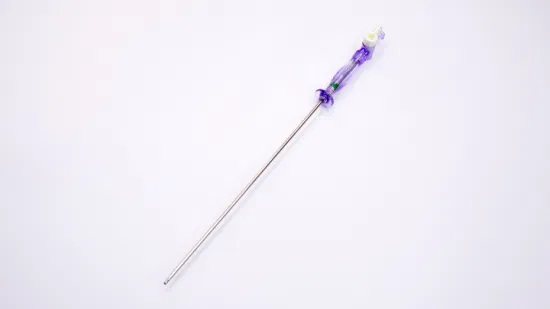
OEM Disposable Laparoscopic Instruments Veress Needles
Overview FDA 510K cleared GTK laparoscopic Pneumoneedles Veress Needles surgical Instruments top China factory Company P
Description
Basic Info.
| Model NO. | VN-120 |
| Custmization | Yes |
| Obese People | Yes |
| Ventilation Capacity | 3L |
| Working Length | 120mm |
| Transport Package | Peel Pouch Package |
| Specification | ISO13485, CE |
| Trademark | TK |
| Origin | China |
| HS Code | 9018321 |
| Production Capacity | 500, 000 PCS/Year |
Product Description
FDA 510K cleared GTK laparoscopic Pneumoneedles Veress Needles surgical Instruments top China factory Company ProfileGTK Medical is a hi-tech medical devices manufacturer of more than 17 years R&D and manufacturing expertise. GTK veress needles are exported to more than 40 countries and used in more than 1000 hospitals worldwide.
G T.K veress needles have been exported to 40 countries, most of which are European and American countries, such as the USA, UK, France, Germany, Italy, Belgium, Spain, Japan, South Korea.
| Veress Needles Specification | |
| Product Code | Working Length (mm) |
| VN-120 | 120 |
| VN-150 | 150 |
| VN-120L | 120 |
| VN-150L | 150 |
| GTK Veress Needle |
| Q: Are you a factory or trading company? |
| A: Yes. We are a factory in China with 17 years surgical devices developing and manufacturing experience. |
| Q: What is the core strength of GTK Medical? |
| A: Our core strength is strong R&D Capability (more than 600 patents applied) and world first class quality (FDA 510K, CE, ISO13485:2016) management on products. |
| Q: What makes GTK Veress Needle stand out? |
| A: Double warning system confirms safe insertion. Ergonomic handle ensures for convenient and easy insertion. |
| Q: Do you provide free samples of GTK Veress Needle for clinical trial use? |
| A: Yes. We are proud and confident to provide free samples for real clinical testing and evaluation. |
| Q: Do you have exported experiences to large medical devices company/companies? |
| A: Yes. We exported to more than 40 countries, most of which are based in the America and Europe. |
| Q: Do you accept factory audit prior to formal partnership? |
| A: Yes. We are proud and confident to accept factory audit. We passed US FDA on site aduits on 2015 and 2021. |
Please do not hesitate to contact me, I am always here waiting for assisting you!
Prev: Strong Polycarbonate Handle Disposable Medical Veress Needle for Laparoscopy Insufflation
Next: Disposable Medical Device Abdominal Surgery Laparoscopic Veress Needle
Our Contact
Send now